Sterile Latex Surgical Gloves – Gammex Beige Size 8.5 (20 Pairs)
$69.00
Out of stock
SKU: 1732271073
Category: Medical Gloves/Masks
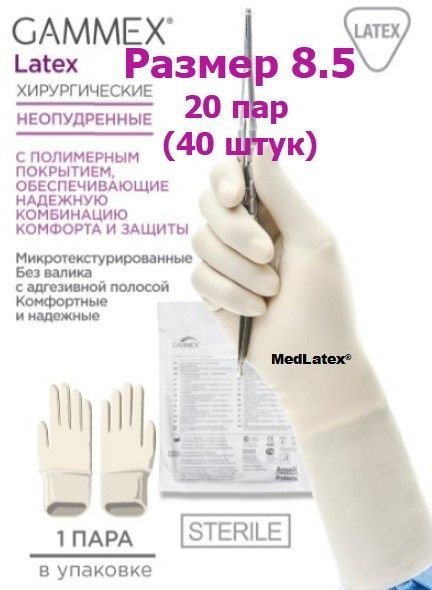
Sterile Latex Surgical Gloves – Gammex Latex Beige Size 8.5 (40pcs)
Experience superior comfort, protection, and sensitivity with Gammex Latex sterile, powder-free surgical gloves. These premium gloves are ideal for all types of surgical procedures, including extended surgeries, and serve as an excellent outer glove in a double-gloving system with puncture indication.
Key Features & Benefits:
- Superior Comfort and Protection: Made from natural latex with a polymer coating, these gloves provide a reliable combination of comfort and protection even during prolonged procedures.
- Enhanced Fit and Feel: The internal polymer coating prevents direct skin contact with natural latex proteins, ensuring quick and easy donning even on damp hands. The micro-textured exterior provides excellent grip and instrument control. The anatomically designed shape, featuring a wider thumb base, minimizes hand fatigue and pressure.
- Secure Fixation: The robust, cuffless design with an adhesive strip prevents slippage and provides a secure fit under surgical gowns.
- Hypoallergenic Properties: Gammex Latex gloves undergo rigorous cleaning and rinsing to minimize free latex proteins, making them suitable for pediatric surgery, obstetrics, gynecology, and patients/staff at risk of latex allergy (including those with a history of atopy or cross-allergies). They are free from mercaptobenzothiazole and thiuram, common causes of contact dermatitis.
- High-Quality Construction: These gloves boast a low protein content (<30 µg/g), a finger thickness of 0.220 mm, palm thickness of 0.200 mm, and cuff thickness of 0.200 mm. The smooth exterior features micro-texturing, and the interior has a DermaShield polymer coating. They offer a medium level of grip.
Specifications:
- Material: Natural rubber latex
- Brand: Ansell Gammex
- Powder: Powder-free
- Color: Beige
- Size: 8.5
- Quantity: 40 gloves (20 pairs)
- Packaging: Individually paired in film pouches, 50 pairs per dispensing box, 200 pairs (4 x 50) per outer carton.
- Compliance: CE Marking MH Class IIa & PPE Category III, AS/NZS 4179, ASTM D6978, ASTM F739, EN 16523-1, EN 374 Micro-organisms, EN 374 Chemical Resistance, EN 420, EN 420:2003 + A1:2009, EN 455-1, EN 455-2, EN 455-3, EN 455-4, EN ISO 374-1, EN ISO 374-1:2016 Type B (KPT), EN ISO 374-5, EN ISO 374-5:2016 VIRUS, ISO 10282, JIS T9107, EN 556, ISO 11137-1, ISO 13485, ISO 14001, ISO 9001, Korean GMP, AQL 0.65.
- Approved for use with: Chemotherapy drugs (according to ASTM D6978; not FDA 510k approved).
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks