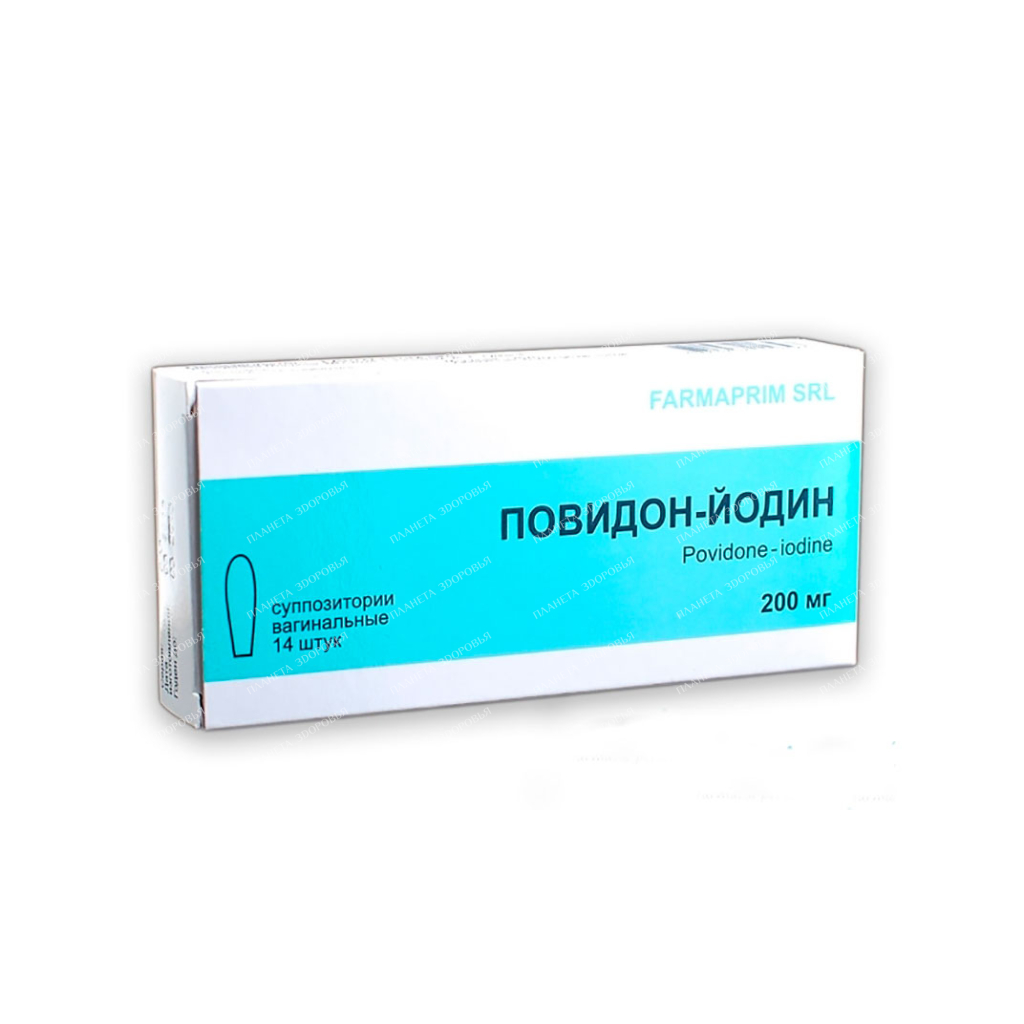
Povidone-Iodine vaginal suppositories 200mg №7×2
$19.00
Name Povidone-iodine. Release form Suppositories MNN Povidone-iodine FTG Antiseptic agent General characteristics Cylindrical-shaped suppositories, brown in color, with a specific odor. Composition One suppository contains: active substance: povidone-iodine – 200 mg; excipients: macrogol 1000. Pharmacotherapeutic group and ATC code Antiseptics and antimicrobials for the treatment of gynecological diseases (excluding combinations with corticosteroids); Other antiseptics and antimicrobials for the treatment of gynecological diseases; G01AX11. Indications for use Vaginitis caused by mixed infections, non-specific infections; fungal infections (Candida albicans), including therapy after antibiotics or steroid drugs; as part of the complex therapy of trichomonas colpitis against the background of the systemic use of nitroimidazole derivatives. If you need more information about your condition, ask your doctor for advice. Method of administration and doses The use of this medicinal product is possible only after consulting a doctor! Do not stop taking Povidone-Iodine without first consulting with your doctor! If you have any doubts or questions, please contact your doctor. For vaginal use. For mild infections, it is recommended to give 1 povidone-iodine vaginal suppository once a day for 7 days, and continue treatment for another 7 days if there is no response or in more severe infections. For treatment-resistant, prolonged vaginal infections, treatment may be extended and 1 suppository may be applied twice daily. How to use The suppository is released from the packaging and, after pre-wetting with water, is inserted deep into the vagina, in the evening, before going to bed. The use of sanitary napkins during treatment is recommended. It is important to pre-moisten the suppository before insertion into the vagina, to ensure optimal dissolution of the active substance, and also to prevent local irritation of the vaginal mucosa. Treatment should not be stopped during menstruation. Adverse reactions Adverse reactions that may develop during treatment are classified according to frequency (MedDRA classification): Very common (≥1/10) Common (≥1/100, <1/10) Uncommon (≥1/1000, < 1/100) Rare (≥1/10,000, <1/1,000) Very rare (<1/10,000) Unknown (The frequency of occurrence cannot be determined from the available data.) Immune system disorders: Rare - hypersensitivity; Very rarely - anaphylactic reactions; Endocrine system disorders: Very rarely - hyperthyroidism (in some cases accompanied by tachycardia and anxiety) *; Metabolic and nutritional disorders: Not known - electrolyte imbalance**, metabolic acidosis**. Skin and subcutaneous tissue disorders: Rarely, contact dermatitis (eg, erythema, small vesicular rash, pruritus); Very rarely - angioedema; Renal and urinary disorders: Not known - acute renal failure**, blood osmolarity disorders**. Injury, poisoning and complications associated with the procedures used: Not known - chemical burns of the skin. *May occur in patients with pre-existing thyroid disorders after using significant amounts of iodine (eg, long-term use of povidone-iodine to treat wounds or burns). ** May occur with long-term use of iodine in significant amounts (for example, for the treatment of burns). Contraindications hypersensitivity to iodine and other components of the drug; hyperthyroidism and other severe thyroid dysfunction; thyroid adenoma; dermatitis herpetiformis Dühring; condition before and after the use of radioactive iodine for the treatment of hypothyroidism (until complete recovery); dysfunction of the thyroid gland (nodular colloid goiter, endemic goiter and Hashimoto's thyroiditis, hyperthyroidism), thyroid adenoma; I trimester of pregnancy; children and adolescents up to 18 years of age. OverdoseSymptoms: For acute iodine poisoning, the following symptoms are characteristic: symptoms of the gastrointestinal tract: metallic taste in the mouth, hypersalivation, burning sensation or pain in the mouth or throat; heaviness in the stomach, diarrhea; irritation and swelling of the eyes; skin reactions; impaired renal function and anuria; circulatory failure; laryngeal edema with secondary asphyxia, pulmonary edema, metabolic acidosis, hypernatremia. Treatment: symptomatic therapy, in particular the correction of electrolyte balance, kidney and thyroid function. Precautions With caution: chronic renal failure. The use of the drug may reduce the absorption of iodine by the thyroid gland, which may affect the results of some studies and procedures (thyroid scintigraphy, determination of protein-bound iodine, diagnostic procedures using radioactive iodine), and therefore, planning the treatment of thyroid diseases with iodine preparations may become impossible . After stopping the use of the drug, an interval of at least 1-4 weeks should be maintained. The oxidizing effect of the drug can lead to false positive results of various diagnostic tests (for example, the determination of hemoglobin and glucose in feces and urine using toluidine and guaiac resins). In the presence of blood, the bactericidal effect may decrease. In case of infection of the sexual partner and to exclude the possibility of reinfection, simultaneous treatment of both partners is mandatory. When the first symptoms of hypersensitivity appear, the drug should be discontinued. The preparation is easily removed from textiles or other materials with warm water and soap. Stubborn stains should be treated with ammonia solution or sodium thiosulfate. During treatment, the use of sanitary pads is recommended. For the treatment of trichomonas colpitis, Povidone-Iodine can be used as a local therapy against the background of the systemic use of nitroimidazole derivatives (metronidazole, tinidazole, ornidazole, secnidazole, etc.). Application for violations of the liver and kidneys Special care is required with regular use of the drug in patients with renal and hepatic insufficiency. Use in the elderly In the case of clinically compensated hyperthyroidism, latent hyperthyroidism and other diseases of the thyroid gland, the drug should be used only under medical supervision. Fertility, pregnancy and lactation Pregnancy and lactation The use of povidone-iodine during pregnancy and lactation is allowed only if it is absolutely necessary, and the period of treatment will be kept to a minimum. Absorbed iodine ions cross the placental barrier and can be excreted into breast milk. Moreover, the fetus and newborn are characterized by hypersensitivity to iodine. Therefore, during pregnancy and lactation, high doses of povidone-iodine should not be used. The level of povidone-iodine in breast milk is higher than the serum level. The use of this medicine may cause transient hyperthyroidism in the fetus or newborn, with elevated levels of thyroid-stimulating hormone. The child's thyroid function may need to be carefully evaluated. Avoid accidental ingestion or accidental contact of the drug with the child's gastrointestinal tract. Reproductive function Vaginal suppositories may have a spermicidal effect, therefore, if pregnancy is planned, the use of the drug Povidone-Iodine is not recommended. The use of the drug in children Not recommended for use in children and adolescents under the age of 18 years, due to the lack of data on efficacy and safety. Influence on the ability to drive vehicles and work with mechanisms The use of the drug does not affect the ability to drive vehicles and control potentially dangerous mechanisms. Interaction with other drugs If you are currently or have recently taken other drugs, tell your doctor. The povidone-iodine complex exhibits a bactericidal effect at a pH in the range of 2.0-7.0. Interaction with proteins and other unsaturated organic compounds reduces the effectiveness of Povidone-Iodine vaginal suppositories. Due to its oxidative properties, povidone-iodine may cause false positive results in some diagnostic tests (eg, fecal and urinary occult blood tests, and to detect glycosuria). Povidone-iodine may reduce thyroid iodine uptake, which may confound some diagnostic tests (eg, thyroid scans, protein-bound iodine, and radioactive iodine), and may interact with iodine medications used to treat thyroid diseases, thus reducing their effectiveness. To obtain reliable results, a sufficiently long break in iodine treatment (1-2 weeks) is recommended before scintigraphy. Patients taking lithium preparations should avoid long-term and regular use of povidone-iodine. Storage conditions Store in a place protected from moisture and light, at a temperature not exceeding 25 ° C. Do not freeze. Shelf life 3 years. Do not use after the expiry date stated on the package. Keep out of the reach of children! Holiday conditions Without a doctor's prescription. PackagingPrimary packaging. Suppositories of 7 pieces in a blister pack made of PVC / PE film. secondary packaging. 2 blister packs No. 7 together with a leaflet in a cardboard box. Buy Povidone-Iodine vaginal suppositories 200mg No. 7x2
| INN | povidone-iodine |
|---|---|
| The code | 4 479 |
| Barcode | 4 840 456 000 393 |
| Dosage | 200mg |
| Active substance | Povidone-iodine |
| Manufacturer | Farmaprim LLC, Moldova |
| Importer | LLC "Iskamed", Republic of Belarus, 220036, Minsk, K. Liebknekhta st., 70, office 6; IOOO "Interfarmaks", Republic of Belarus, 223028, Minsk region, Minsk district, Zhdanovichsky s / s, ag. Zhdanovichi, st. Zvezdnaya, 19A-5, pom. 5-2 |
Related products
Antifungals & Antiparasitics
Antifungals & Antiparasitics
Antifungals & Antiparasitics
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks