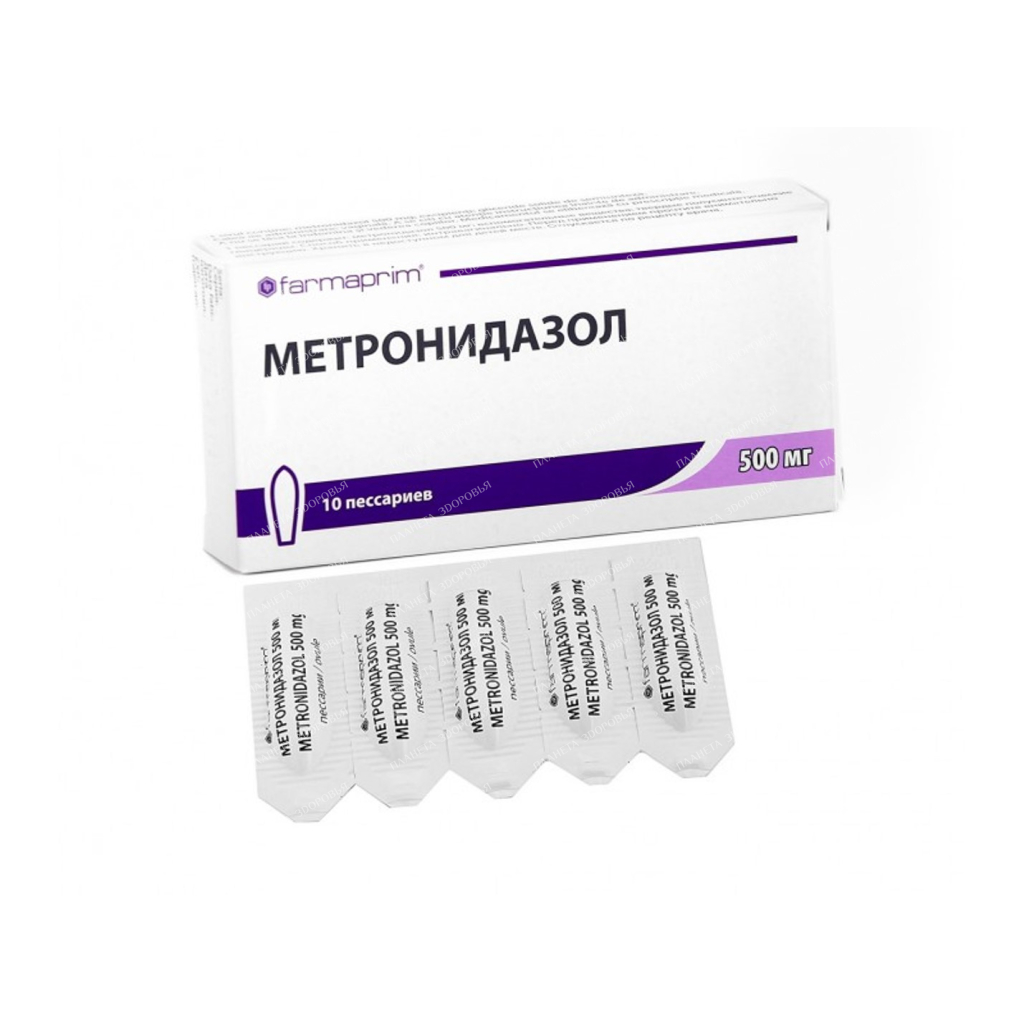
Metronidazole vaginal suppositories 500mg №5×2
$19.00
Name:
Metronidazole supp.vag 500mg in a cell.pack No. 5×2
Description:
Suppositories are cylindrical in shape, white or white with a yellowish tint. On the cut, the presence of an air and porous rod, and a funnel-shaped recess is allowed. The main active substance Metronidazole Product form Vaginal suppositories Dosage 500 mg Pharmacological action Antiseptics and antimicrobials for the treatment of gynecological diseases. imidazole derivatives. ATX code: G01AF01. Indications for use Local treatment of trichomonas vaginitis, bacterial vaginosis and non-specific vaginitis caused by microorganisms sensitive to metronidazole. If you need more information about your condition, ask your doctor for advice. Method of administration and doses The use of this medicinal product is possible only after consulting a doctor! Do not stop taking Metronidazole without first consulting with your doctor! If you have any doubts or questions, please contact your doctor. Intravaginally. The drug is allowed to be used for the treatment of adult patients only. Metronidazole vaginal suppositories are usually used with metronidazole in tablet form. Trichomonas vaginitis Assign 1 vaginal suppository 1 time per day for 10 days. The suppository is inserted deep into the vagina. Treatment should be carried out with simultaneous oral administration of metronidazole tablets: 1 tablet (250 mg) twice a day for 10 days. Nonspecific vaginitis 1 vaginal suppository is injected deep into the vagina 1 time per day for 7 days. The maximum duration of treatment should not exceed 10 days, and the number of courses of treatment should not exceed 3 per year. How to use suppositories Suppositories are administered intravaginally. Suppositories should not be cut into pieces, since such changes in the storage of the drug may lead to a violation of the distribution of the active substance. Use during pregnancy and lactation If you are pregnant or breastfeeding, if you suspect that you are pregnant or do not exclude the possibility of pregnancy, inform your doctor. In the first trimester of pregnancy and during lactation, the drug is contraindicated. In the II and III trimesters of pregnancy, the drug can be used only if the expected benefit to the mother outweighs the potential risk to the fetus and under medical supervision. Precautions Long-term use of the drug requires monitoring of the blood count. If a patient develops leukopenia, it is important to carefully balance the expected benefit of continuing treatment with the possible risk. It is necessary to be aware of the risk of deterioration in the neurological status of patients with severe, chronic or acute neurological diseases in the treatment of metronidazole. Patients with permanent or progressive neuropathies should be given metronidazole very carefully. It is necessary to stop treatment if ataxia, dizziness, hallucinations appear, and if the patient’s neurological status worsens. Metronidazole is able to immobilize treponemas, resulting in a false positive Nelson test. During treatment with metronidazole, alcohol should be avoided, as tachycardia, vomiting, and a feeling of heat may occur. With vaginitis caused by Trichomonas vaginalis, simultaneous treatment of sexual partners is advisable, treatment of a partner is recommended with metronidazole for oral administration. Use of the drug in children The drug is contraindicated in children under 18 years of age. Interaction with other drugs If you are currently or have recently taken other drugs, tell your doctor. When used simultaneously with indirect anticoagulants (for example, warfarin), metronidazole enhances their effect and the risk of bleeding by reducing its metabolism in the liver, which leads to an increase in prothrombin time. More frequent monitoring of prothrombin time and INR monitoring is needed, dose adjustment of oral anticoagulants during treatment with metronidazole and 8 days after withdrawal. It is not recommended to combine with non-depolarizing muscle relaxants (for example, vecuronium bromide). Similar to disulfiram, it causes ethanol intolerance. The simultaneous use of metronidazole with disulfiram can lead to the development of various neurological syndromes (depression of consciousness, the development of mental disorders). Under the influence of barbiturates (for example, phenobarbital), the effectiveness of metronidazole is reduced by accelerating its inactivation in the liver. With simultaneous use with cimetidine, the level of metronidazole in the blood serum may increase and the risk of adverse reactions increases. When taken simultaneously with lithium preparations, the concentration of the latter in plasma may increase. With simultaneous use with fluorouracil, the toxic effect is enhanced, but not the effectiveness of fluorouracil. Contraindications Hypersensitivity to metronidazole or other nitroimidazole derivatives; blood diseases, leukopenia (including history); impaired coordination of movements, diseases of the central nervous system (including epilepsy); liver failure (when prescribing large doses); pregnancy (I trimester), lactation, children under 18 years of age. With caution: pregnancy (II-III trimesters). Composition One suppository contains: active substance: metronidazole 500 mg; excipients: solid fat. Overdose Symptoms: leukopenia, neuropathy, ataxia, vomiting. Since no specific antidote for metronidazole is known, symptomatic therapy is recommended. Adverse reactions Gastrointestinal disorders: epigastric pain, nausea, vomiting, diarrhea, inflammation of the oral mucosa, taste disorders, anorexia, exceptional cases of pancreatitis, which are reversible. Skin changes: rash, itching, redness, urticaria, fever, angioedema; very rarely – exceptional cases of anaphylactic shock; in isolated cases – pustular rash. Central and peripheral nervous system disorders: peripheral sensory neuropathy, headache, convulsions, dizziness, very rarely cases of encephalopathy (eg, confusion) and subacute cerebellar syndrome (eg, ataxia, dysarthria, gait disturbance, nystagmus, tremor), which may disappear after discontinuation of the drug. Mental disorders: psychotic disorders, including confusion, hallucinations. Visual impairment: temporary visual impairment, such as diplopia, myopia. Hematology: in isolated cases – agranulocytosis, neutropenia and thrombocytopenia. Violation of liver function: in isolated cases – a deviation from the norm of liver function tests, which are reversible, cholestatic hepatitis. During treatment, urine may acquire a red-brown color, which is due to the presence of water-soluble pigments, which are a metabolic product of metronidazole. Storage conditions In a dry, dark place, at a temperature not exceeding 25°C. Do not freeze. Buy Metronidazole vaginal suppositories 500mg №5×2 Price for Metronidazole vaginal suppositories 500mg №5×2
| INN | OTHER |
|---|---|
| The code | 4 477 |
| Barcode | 4 840 456 000 355 |
| Dosage | 500mg |
| Active substance | Metronidazole |
| Manufacturer | Farmaprim LLC, Moldova |
| Importer | IOOO Interfarmaks 223028 Minsk region, Minsk district, Zhdanovichsky s / s, ag. Zhdanovichi, st. Star, 19a-5, room. 5-2 |
Related products
Antifungals & Antiparasitics
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks