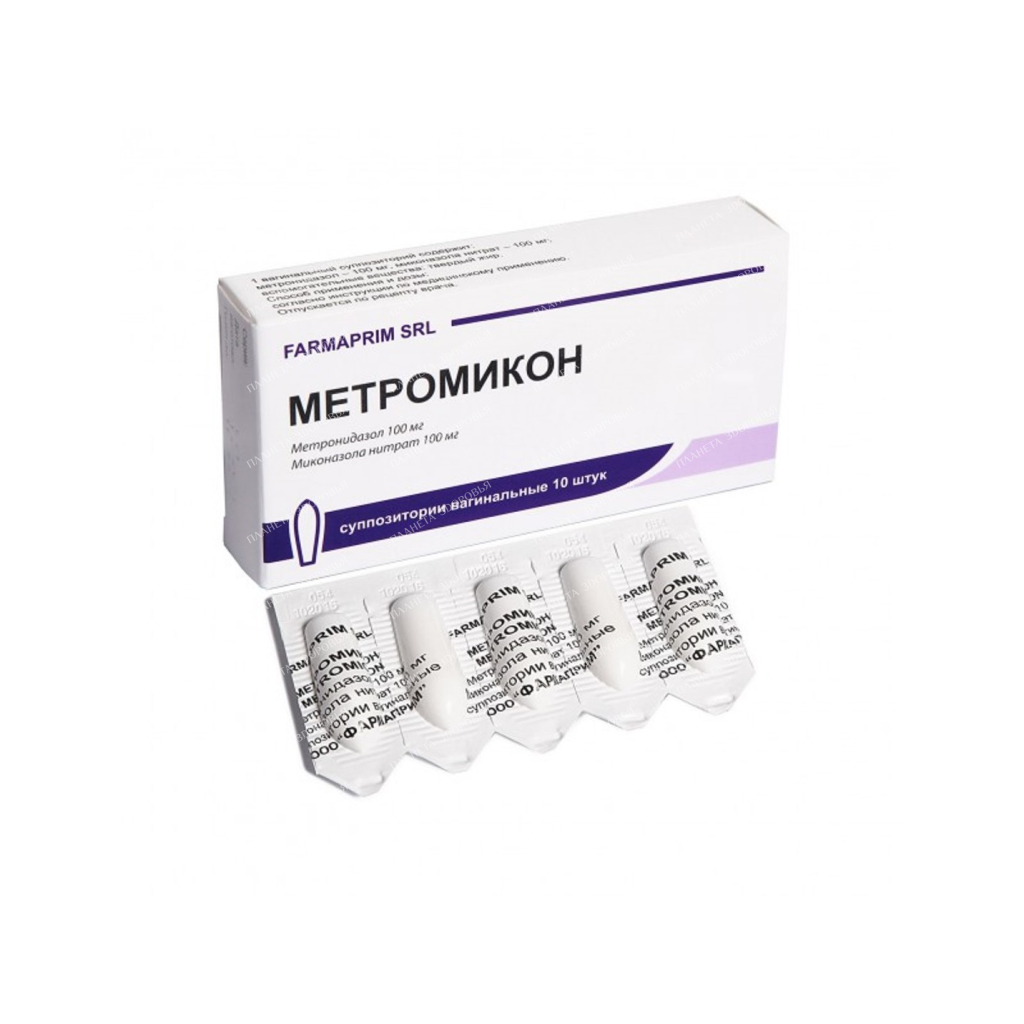
Metromicon vaginal suppositories 100mg/100mg №5×2
$29.00
NameMetromicon supp.vag.100mg/100mg in cc.cell.pack No. 5×2 On the cut, the presence of an air and porous rod, and a funnel-shaped recess is allowed. The main active substance Metronidazole Release form Suppositories Dosage 100 mg / 100 mg Pharmacological action Antiseptics and antimicrobials for the treatment of gynecological diseases. Combinations of imidazole derivatives. ATX code: G01AF20. Indications for use Local treatment of vaginal infections: vaginal candidiasis; trichomonas vulvovaginitis; bacterial vaginosis; vaginitis caused by mixed infections. Method of administration and doses The use of this medicinal product is possible only after consulting a doctor! Do not stop taking Metromicon without first consulting with your doctor! If you have any doubts or questions, please contact your doctor. Intravaginally. With trichomoniasis, bacterial vaginosis for 10 days, once a day (in the evening before going to bed), 1 Metromicon suppository is injected deep into the vagina. With trichomoniasis, systemic metronidazole preparations are simultaneously used. It is advisable to treat the sexual partner with systemic metronidazole preparations. If necessary, the course of treatment can be repeated. With vulvovaginal candidiasis, 1 suppository Metromicon is injected deep into the vagina for 10 days, 1 time per day (in the evening before bedtime). Use during pregnancy and lactation If you are pregnant or breastfeeding, if you suspect that you are pregnant or do not rule out the possibility of pregnancy, tell your doctor about it. The use of Metromicon in the II and III trimesters of pregnancy is possible only under strict indications when the intended benefit to the mother outweighs the potential risk to the fetus. If it is necessary to prescribe the drug during lactation, breastfeeding should be discontinued, since metronidazole is excreted in breast milk. Breastfeeding can be resumed 24-48 hours after the end of treatment. Precautions Be wary in patients with hematopoietic disorders, diseases of the peripheral and central nervous system, renal and hepatic insufficiency. With indications in the anamnesis of changes in the composition of peripheral blood, as well as when using the drug in high doses and / or with prolonged use, it is necessary to control the picture of peripheral blood (danger of leukopenia). Any deterioration in the patient’s neurological status requires discontinuation of treatment. During treatment, it is possible to change the results when determining liver enzymes in the blood and glucose. Metronidazole can immobilize treponemes, resulting in a false positive TPI test (Nelson’s treponemal test). When using the drug should refrain from sexual intercourse. In order to prevent re-infection, simultaneous treatment of the sexual partner is necessary, in the case of trichomonas vaginitis, in combination with metronidazole intake. The use of vaginal suppositories can reduce the reliability of mechanical contraception (condoms, vaginal diaphragms) due to the interaction of the suppository base with latex or rubber. During treatment and for 24-48 hours after the end of the course of treatment, it is forbidden to drink alcoholic beverages and take drugs that contain ethanol (due to the possible occurrence of a disulfiram-like reaction). Use in children: it is not recommended to use the drug in virgins and in children under 18 years of age. Use by elderly patients: due to age-related changes in the pharmacokinetics of metronidazole in elderly patients, dose adjustment of the drug may be required, especially when used simultaneously with systemic action of metronidazole. Interaction with other drugs If you are currently or have recently taken other drugs, tell your doctor. With the simultaneous use of metronidazole enhances the effect of indirect anticoagulants. Prothrombin time may increase, so dose adjustment of indirect anticoagulants is necessary. With the simultaneous use of metronidazole with disulfiram, various neurological symptoms may develop; with phenytoin, the level of phenytoin in the blood increases, and the level of metronidazole in the blood decreases; with lithium preparations, an increase in the toxicity of the latter can be observed; with phenobarbital, the level of metronidazole in the blood decreases; with cimetidine, the level of metronidazole in the blood may increase. Metronidazole and miconazole nitrate inhibit the metabolism of astemizole and terfenadine, resulting in increased plasma concentrations of astemizole and terfenadine. It is possible to change the concentration of theophylline and procainamide in the blood plasma with simultaneous use with Metromicon. Contraindications Hypersensitivity to the components of the drug or to other derivatives of nitroimidazole; epilepsy; porphyria; severe liver dysfunction; 1 trimester of pregnancy, lactation period; children’s age up to 18 years; virginity. Composition 1 suppository contains: active substances: metronidazole 100 mg, miconazole nitrate 100 mg; excipients: solid fat. Overdose There are no data on overdose with intravaginal use of metronidazole. However, with simultaneous use with metronidazole, systemic effects may develop inside. Symptoms of an overdose of metronidazole: nausea, vomiting, abdominal pain, diarrhea, itching, metallic taste in the mouth, ataxia, dizziness, paresthesia, convulsions, leukopenia, dark staining of urine. Symptoms of an overdose of miconazole have not been identified. Treatment: symptomatic and supportive therapy is recommended, in case of accidental ingestion – gastric lavage. Side effects If side effects occur, tell your doctor about it. This applies to all possible side effects, including those not described in this package insert. MedDRA convention by frequency Very common (? 1/10); frequent (? 1/100, < 1/10); infrequent (? 1/1000, < 1/100); rare (? 1/10,000, < 1/1000); very rare (< 1/10,000), frequency unknown (cannot be estimated from the available data). With vaginal use of the drug, side effects occur rarely (> 1/10,000 and < 1/1000) or very rarely (< 1/10,000) due to minimal plasma concentrations. Gastrointestinal disorders: Abdominal pain or cramps, metallic taste, dry mouth, constipation, diarrhea, loss of appetite, nausea, vomiting. Nervous system disorders: headache, movement disorders (ataxia), dizziness, psycho-emotional disorders, peripheral neuropathy (with prolonged use of the drug), convulsions. Hematopoietic system disorders: leukopenia. From the genitourinary system: staining of urine in a red-brown color, due to the presence of a water-soluble pigment resulting from the metabolism of metronidazole. Allergic reactions: urticaria, itching of the skin, rash. Local reactions: itching, burning, pain and irritation of the vaginal mucosa, especially at the beginning of treatment, which does not require discontinuation of treatment and is determined by the effect of the drug on the inflamed vaginal mucosa. If irritation is severe, treatment should be discontinued. Storage conditionsStore in a dry, dark place at a temperature not exceeding 25 °C. Do not freeze. Shelf life - 3 years. Buy Metromicon vaginal suppositories 100mg/100mg №5x2 Price for Metromicon vaginal suppositories 100mg/100mg №5x2
| INN | METRONIDAZOL+MICONAZOL |
|---|---|
| The code | 4 739 |
| Barcode | 4 840 456 000 430 |
| Dosage | 100mg/100mg |
| Active substance | metronidazole, miconazole |
| Manufacturer | Farmaprim LLC, Moldova |
| Importer | IOOO Interfarmaks 223028 Minsk region, Minsk district, Zhdanovichsky s / s, ag. Zhdanovichi, st. Star, 19a-5, room. 5-2 |
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks