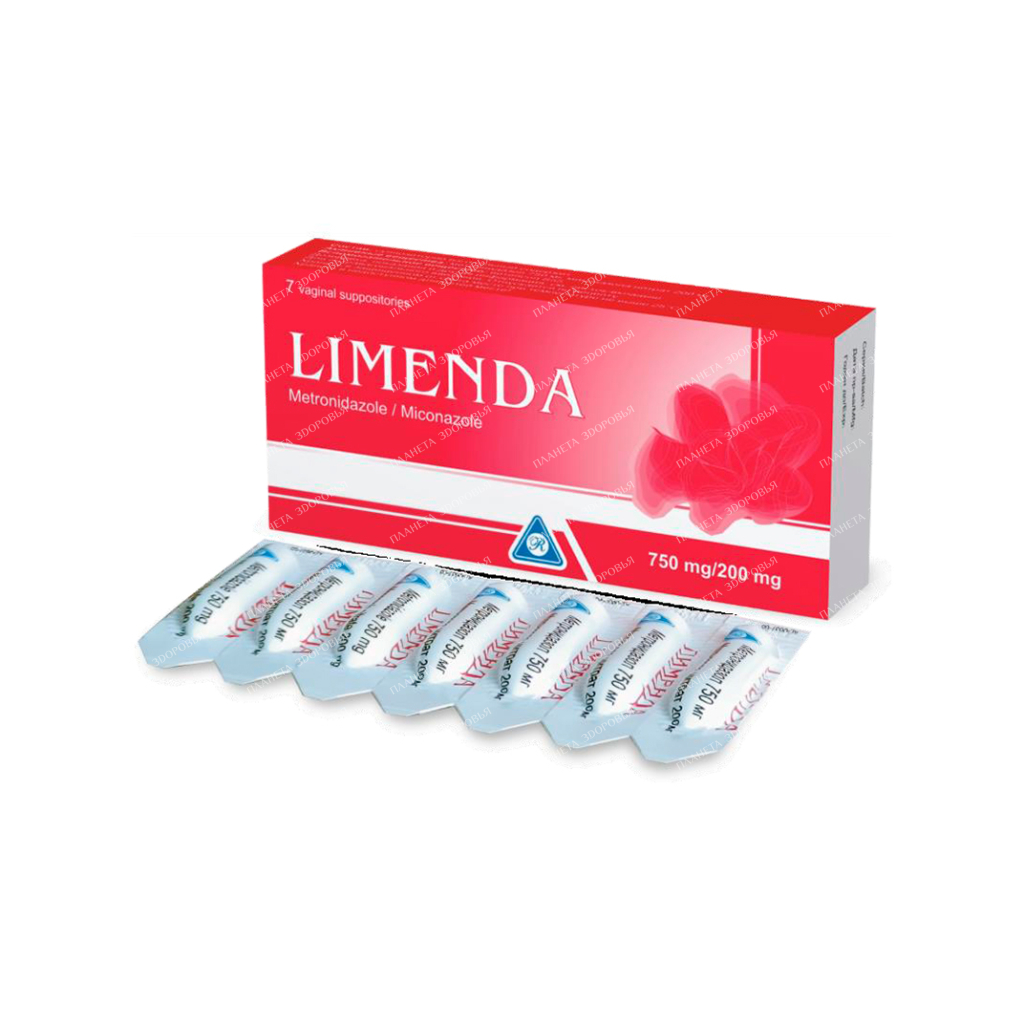
Limenda vaginal suppositories 750mg/200mg №7×1
$39.00
Name Limenda sup.vag. 750mg200mg in bl. in pack. No. 7×1 Dosage form Vaginal suppositories Composition One suppository contains active substances: metronidazole 750 mg, miconazole nitrate 200 mg, excipient – witepsol S55 Antiseptics and antimicrobials for the treatment of gynecological diseases (excluding combinations with corticosteroids). imidazole derivatives. Imidazole derivatives combination. ATC code G01AF20 Pharmacokinetics The bioavailability of metronidazole with intravaginal use is 20% compared with oral administration. After vaginal administration, upon reaching the equilibrium state, the plasma concentration of metronidazole was 1.6-7.2 μg / ml. Systemic absorption of miconazole nitrate with this route of administration is very low (approximately 1.4% of the dose), miconazole nitrate in plasma was not detected. Metronidazole is metabolized in the liver. The hydroxyl metabolite is active. The half-life of metronidazole is 6-11 hours. Approximately 20% of the dose is excreted unchanged by the kidneys. Pharmacodynamics Limenda is a combined antimicrobial drug, the action of which is due to the constituents of metronidazole and miconazole. Metronidazole is an antimicrobial and antiprotozoal agent effective against Gardnerella vaginalis and anaerobic bacteria, including anaerobic streptococci and Trichomonas vaginalis. Miconazole nitrate has a wide spectrum of activity and is especially effective against pathogenic fungi, including Candida albicans, in addition, miconazole nitrate is effective against gram-positive bacteria. Indications for the use of vaginal candidiasis, bacterial vaginosis, trichomonas vaginitis, caused by mixed infections. Dosage and administration The drug is administered intravaginally, 1 suppository at night for 7 days. With recurrent vaginitis or vaginitis resistant to other treatments, the course of treatment can be increased up to 14 days. Vaginal suppositories should be inserted deep into the vagina using the disposable fingertips contained in the package. For elderly patients over 65 years of age, dosage adjustment is not required. Side effects Rarely hypersensitivity reactions (skin rash) headache or cramps in the abdomen vaginal itching, burning and irritation of the vagina Very rarely leukopenia fatigue, dizziness change in taste sensations, constipation, dry mouth, metallic taste diarrhea, loss of appetite, nausea, vomiting movement disorders (ataxia), peripheral neuropathy (with prolonged use of the drug and overdose), convulsions When combined with phenytoin, there is a decrease in the concentration of metronidazole in the blood while increasing the concentration of phenytoin. When co-administered with cimetidine, the concentration of metronidazole in the blood may increase and the risk of developing neurological side effects may increase. Co-administration with lithium may increase the toxicity of lithium. Limenda increases plasma concentrations of astemizole and terfenadine. The possible interaction of metronidazole with alcohol can cause disulfiram-like reactions. It is possible to change the results when determining the level of liver enzymes, glucose (hexokinase method), theophylline and procainamide in the blood. Special instructions Do not take orally. For intravaginal use only. Limenda is not recommended for use in virgins. It is necessary to avoid alcohol during treatment and at least for 24-48 hours after the end of the course due to possible disulfiram-like reactions. Caution should be exercised when using suppositories simultaneously with contraceptive diaphragms and condoms due to possible damage to the rubber base of the suppositories. In patients with trichomonas vaginitis, simultaneous treatment of the sexual partner is necessary. It is possible to change the results when determining the level of liver enzymes, glucose (hexokinase method), theophylline and procainamide in the blood. Pediatric use Not recommended for children and adolescents under 18 years of age due to insufficient data on use in this age group. Pregnancy and lactation In the II and III trimester of pregnancy, the drug is prescribed only if other methods of treatment are ineffective, taking into account the benefit / risk ratio. At the time of treatment, breastfeeding should be stopped, since metronidazole passes into breast milk. Breastfeeding can be resumed 24-48 hours after the end of treatment. Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms The drug does not affect the ability to drive vehicles and control mechanisms. Overdose There are no data on overdose with intravaginal use of metronidazole. After intravaginal administration, metronidazole may be absorbed in sufficient amounts to cause systemic effects. Symptoms: nausea, vomiting, abdominal pain, diarrhea, generalized itching, metallic taste in the mouth, movement disorders (ataxia), dizziness, paresthesia, convulsions, peripheral neuropathy (including after prolonged use in high doses), leukopenia, dark urine. Treatment: in case of accidental ingestion, gastric lavage is necessary. There is no specific antidote. Symptomatic and supportive therapy is recommended. Release form and packaging 7 suppositories in a PVC/PE blister pack. 1 or 2 blister packs, together with a pack of fingertips (7 or 14 pieces, respectively) and instructions for medical use in the state and Russian languages, are placed in a cardboard pack. Storage conditionsKeep in a place protected from light at a temperature not exceeding 25°C. Keep out of the reach of children! Shelf life 3 years Do not use after the expiration date. Conditions for dispensing from pharmacies By prescription Buy Limenda vaginal suppositories 750mg/200mg No. 7×1 Price for Limenda vaginal suppositories 750mg/200mg No. 7×1
| INN | METRONIDAZOL+MICONAZOL |
|---|---|
| The code | 146 387 |
| Barcode | 8 680 199 900 712 |
| Active substance | metronidazole, miconazole |
| Manufacturer | World Medicine Ilach Sun. Ve Tij.F.Sh, Turkey |
| Importer | Production and trade private unitary enterprise "NOVAMEDIKA", 223017 Minsk district, Gatovo village, Metallurgicheskaya street, 16a-3; Limited Liability Company "VitVar", 210023, Vitebsk, Frunze Ave., 26, building 3; Closed joint-stock company "UNIPHARM", 223060, Minsk region, Novodvorsky s / council, 40-1, room. 36, area of the village of Bolshoye Stiklevo; Limited Liability Company "COMFARM", 220131 Minsk, Sosnovy Bor st., 4, office 1; [x] Production and trade private unitary enterprise "NOVAMEDIKA", 223017 Minsk district, Gatovo settlement, Metallurgicheskaya st., 16a-3; Limited Liability Company "VitVar", 210023, Vitebsk, Frunze Ave., 26, building 3; Closed joint-stock company "UNIPHARM", 223060, Minsk region, Novodvorsky s / council, 40-1, room. 36, area of the village of Bolshoye Stiklevo; Limited Liability Company "COMFARM", 220131 Minsk, Sosnovy Bor st., 4, office 1 |
Related products
Antifungals & Antiparasitics
Antifungals & Antiparasitics
Antifungals & Antiparasitics
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks