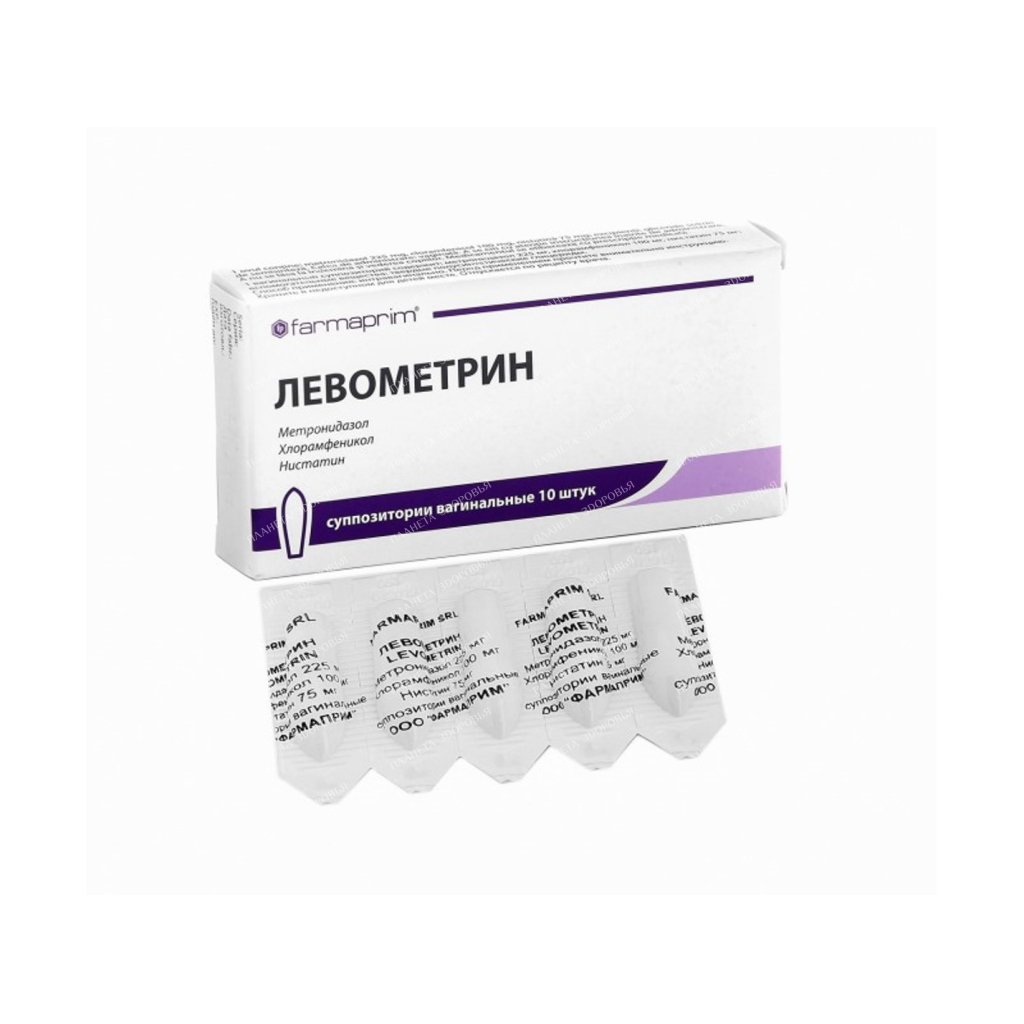
Name:
Levometrin supp.vag.in kont.cell. unitary enterprise No. 5х2
Description:
Suppositories of a cylindric form, yellow color. On the cut, the presence of an air and porous rod, and a funnel-shaped recess is allowed. The main active substance Metronidazole Release form Suppositories Pharmacological action Antiseptics and antimicrobials for the treatment of gynecological diseases. ATC code: G01A X. Indications for use – As an adjunct to systemic therapy for urogenital trichomoniasis. – Local therapy of bacterial vaginosis, candidal colpitis, mixed vaginal infection caused by sensitive microflora. If you need more information about your condition, ask your doctor for advice. Method of application and doses The use of this medicinal product is possible only after consulting a doctor! Do not stop taking Levometrin without first consulting with your doctor! If you have any doubts or questions, please contact your doctor. Intravaginally. 1 suppository 2 times a day (morning and evening). In the treatment of trichomonas vaginitis, Levometrin suppositories must be combined with oral forms of metronidazole or other systemic trichomonacid preparations. At the same time, it is necessary to treat the sexual partner with trichomonacid drugs of systemic action. Use during pregnancy and lactation If you are pregnant or breastfeeding, if you suspect that you are pregnant or do not exclude the possibility of pregnancy, inform your doctor. The drug is contraindicated in the first trimester of pregnancy and lactation. Application in the II and III trimesters of pregnancy is possible only if the potential benefit to the mother outweighs the possible risk to the fetus. If necessary, the appointment of the drug during lactation, breastfeeding should be discontinued. Breastfeeding can be resumed 24-48 hours after the end of treatment. Precautions In the event of adverse reactions, inform your doctor. This applies to all possible adverse reactions, including those not described in this package insert. General disorders and disorders at the injection site: burning, itching, especially at the beginning of treatment, which, however, do not require discontinuation of treatment and are determined by the effect of the drug on the irritated vaginal mucosa. Gastrointestinal disorders: Abdominal pain and cramps, metallic taste, dry mouth, constipation, diarrhea, loss of appetite, nausea, vomiting. Nervous system disorders: headache, movement disorders (ataxia), dizziness, psycho-emotional disorders, peripheral neuropathy (with prolonged use of the drug), convulsions. Blood and lymphatic system disorders: leukopenia. Immune system disorders: skin rashes, incl. hives. With caution in severe violations of liver function (including porphyria), impaired hematopoiesis and diseases of the peripheral and central nervous system. When using the drug should refrain from sexual intercourse. The use of suppositories can reduce the reliability of mechanical contraception (condoms, vaginal diaphragms) due to the interaction of the suppository base with latex or rubber. In order to prevent re-infection, simultaneous treatment of the sexual partner is necessary, and in the case of trichomonas vaginitis, treatment of the sexual partner with trichomonacid preparations of systemic action is necessary. During treatment and within 24-48 hours after the end of the course of treatment, alcohol should not be consumed due to the possibility of developing alcohol intolerance (disulfiram-like reactions). When using the drug, there may be a change in the level of liver enzymes in the blood and glucose (when determined by the hexokinase method). Do not take orally or otherwise than by intravaginal route. High doses and long-term systemic use of metronidazole can cause peripheral neuropathy and epilepsy. Patients with renal / hepatic insufficiency Renal insufficiency: the half-life does not change. Dose reduction is not required. However, in severe cases requiring hemodialysis, dose adjustment is necessary. In cases of severe hepatic insufficiency, the clearance of metronidazole may be impaired. At high levels of metronidazole in blood plasma, an increase in the symptoms of encephalopathy can be observed, so metronidazole should be used with caution in patients with hepatic encephalopathy. The daily dose in patients with hepatic encephalopathy should be reduced to 1/3. Use in pediatrics: the drug can be prescribed from 18 years of age. Interaction with other drugs When used simultaneously with: – indirect anticoagulants – enhances their effect; – ethanol – disulfiram-like reactions can be observed; – disulfiram – disturbances from the central nervous system can be observed; – phenytoin – the level of phenytoin in the blood increases, and the level of metronidazole in the blood decreases; – lithium preparations – there may be an increase in their toxicity; – phenobarbital – the level of metronidazole in the blood decreases; – cimetidine – the level of metronidazole in the blood may increase; Contraindications – hypersensitivity to the components of the drug; – severe liver dysfunction; – epilepsy; – porphyria; – I trimester of pregnancy; – in patients who drink alcohol during treatment or plan to use it within 3 days after the end of the course of treatment; – in patients taking disulfiram during treatment or planning to use it within 2 weeks after the end of treatment. One suppository contains: active substances: metronidazole 225 mg, chloramphenicol 100 mg, nystatin 75 mg (330,000 IU); excipients: semi-synthetic glycerides – up to 2.0 g. Overdose So far, no cases of overdose have been reported. Possible symptoms: nausea, vomiting, abdominal pain, diarrhea, itching, metallic taste in the mouth, ataxia, headache, dizziness, paresthesia, convulsions, leukopenia, dark urine staining (due to an overdose of metronidazole). Treatment: symptomatic therapy, in case of accidental ingestion – gastric lavage. Side effects From the gastrointestinal tract: pain and cramps in the abdomen, metallic taste, dry mouth, constipation, diarrhea, loss of appetite, nausea, vomiting; From the nervous system: headache, motor disorders (ataxia), dizziness, psychoemotional disorders, peripheral neuropathy (with prolonged use of the drug), convulsions; From the hemopoietic system: leukopenia; Allergic reactions: skin rashes, incl. hives; Local reactions: burning, itching, especially at the beginning of treatment, which, however, do not require discontinuation of treatment and are determined by the effect of the drug on the irritated vaginal mucosa. Storage conditions Store in a dry, dark place at a temperature of 15 to 25 °C. Keep out of the reach of children! Buy Levometrin vaginal suppositories No. 5×2 Price for Levometrin vaginal suppositories No. 5×2 Instructions for use for Levometrin vaginal suppositories No. 5×2
| INN | METRONIDAZOLE+CHLORAMPHENICOL+NYSTATIN |
|---|---|
| The code | 24 281 |
| Barcode | 4 840 456 000 584 |
| Active substance | Metronidazole, chloramphenicol, nystatin |
| Manufacturer | Farmaprim LLC, Moldova |
| Importer | IOOO Interfarmaks 223028 Minsk region, Minsk district, Zhdanovichsky s / s, ag. Zhdanovichi, st. Star, 19a-5, room. 5-2 |
Related products
Antifungals & Antiparasitics
Antifungals & Antiparasitics
Antifungals & Antiparasitics
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks