Name:
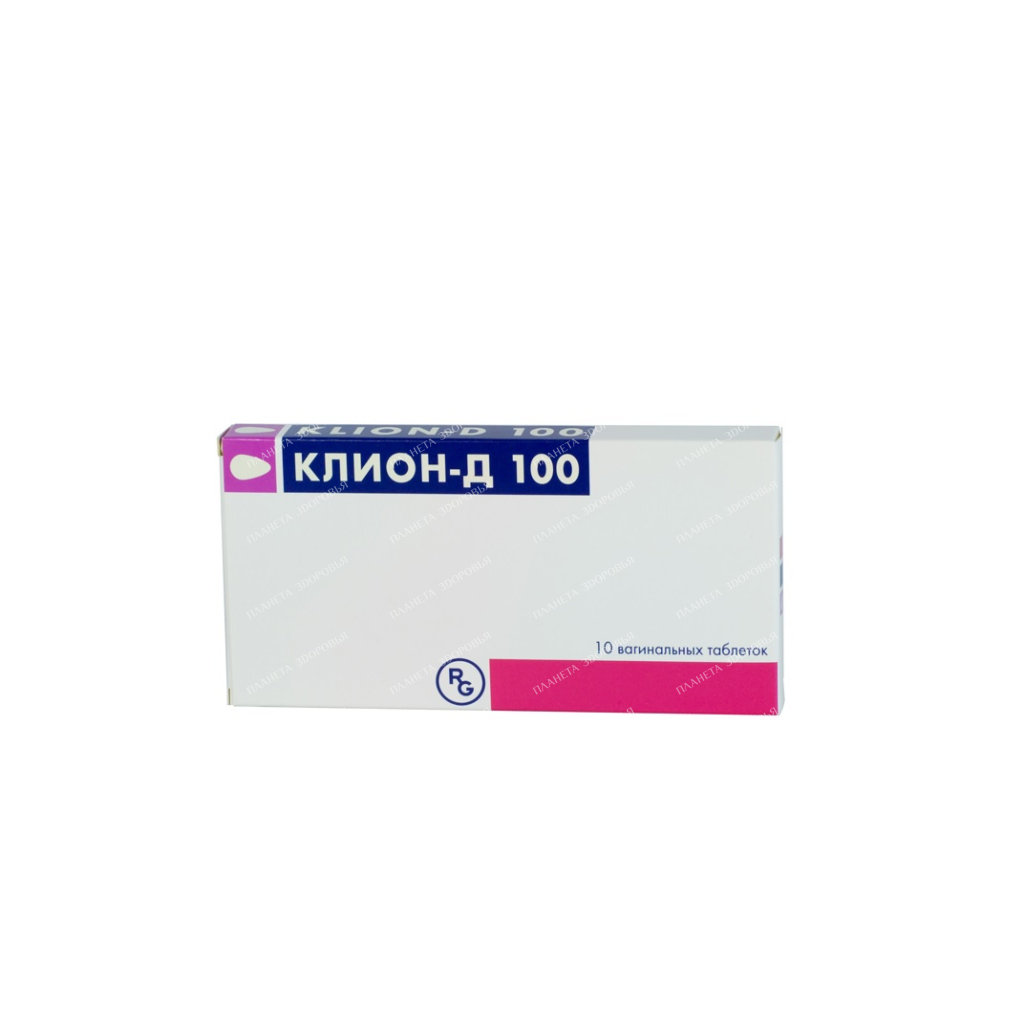
Klion-D 100 tab.vag. 100mg/100mg in bl. in pack. No. 10×1
Description:
Biconvex vaginal tablets, oval in shape with a pointed end, almost white, about 24 mm in size? 14 mm, engraved with “100” on one side. The main active substance Metronidazole + miconazole Release form Tablets Dosage 100 mg Pharmacological properties Pharmacodynamics Metronidazole is a drug for the treatment of trichomoniasis for topical and oral use. Miconazole nitrate is an effective antifungal drug that is used to treat infections caused by dermatophytes and Candida; in addition, when applied topically, it has a pronounced bacteriostatic effect against a number of gram-positive bacteria. The purpose of the topical application of this combined preparation is the local treatment of trichomoniasis, as well as the prevention of vaginal mycosis, which often occurs after treatment with metronidazole. Also, the drug can be used for vaginal mycoses not associated with metronidazole treatment. Pharmacokinetics With intravaginal use, metronidazole and miconazole nitrate are slightly absorbed. According to studies, the level of metronidazole in serum is 0.2 μg / ml, the level of miconazole nitrate is 0.3 μg / ml. Oral metronidazole is usually well absorbed and peak plasma concentrations are reached between the first and third hours. With a single oral dose of 250 mg, a peak plasma concentration of 5 μg / ml is achieved, determined by gas chromatography. The bioavailability of the drug when taken orally is almost 100%. According to studies conducted with the participation of healthy volunteers and patients, metronidazole quickly penetrates into the cerebrospinal fluid and reaches therapeutic concentrations in brain abscesses and lung abscesses. It has a significant volume of distribution and less than 20% of circulating metronidazole is bound to plasma proteins. The drug enters the biliary tract, where concentrations comparable to plasma levels are reached. The mean half-life of metronidazole in healthy volunteers is 8 hours. The main route of excretion of metronidazole and its metabolites is urine (60-80% of the dose), 6-15% of the dose is excreted in the feces. Indications for use Local treatment of vaginitis caused by Trichomonas and / or fungal infection Dosage and administration For trichomoniasis: simultaneously with taking metronidazole orally, 1 vaginal tablet of Klion-D 100, slightly moistened beforehand, must be injected deep into the vagina once a day (in the evening before bedtime ) within 10 days. During these 10 days, you must take 2 tablets of metronidazole (250 mg x 2) daily by mouth (one tablet in the morning and one in the evening) during or after meals, without chewing. It is advisable to simultaneously treat the sexual partner with metronidazole tablets for oral administration. In case of treatment failure: the 10-day course of treatment can be repeated. For candidiasis: 1 vaginal tablet of Klion-D 100, slightly moistened beforehand, must be injected deep into the vagina once a day (in the evening before going to bed) for 10 days. Use during pregnancy and lactation Pregnancy The drug is contraindicated in the first trimester of pregnancy. Metronidazole, when taken orally, crosses the placental barrier and rapidly enters the fetal circulation. Reproductive toxicity studies in rats have used doses five times higher than those used in humans. In the course of these studies, no signs of impaired fertility or damage to the fetus under the influence of metronidazole were detected. When intraperitoneally administered to mice at a dose approximately corresponding to that used in humans, metronidazole had a fetotoxic effect. However, when administered orally to pregnant mice, no fetotoxic effect was observed. However, there are currently no adequate and well-controlled studies in pregnant women. Based on a meta-analysis of studies conducted in the first trimester of pregnancy, it was concluded that no increase in fetotoxicity was observed. Despite this, metronidazole can be used during pregnancy only if (and especially in the first trimester of pregnancy) if the expected benefit outweighs the possible risks. Breast-feeding Metronidazole, when taken orally, is excreted into breast milk at concentrations similar to plasma. It can give breast milk a bitter taste. To protect the infant from the effects of the drug, stop taking metronidazole, or stop breastfeeding while taking metronidazole, as well as within 1 or 2 days after the end of therapy, taking into account the need for therapy for the mother. Precautions During the course of treatment with Klion-D 100, and also for at least one day after its completion, the use of alcoholic beverages is prohibited. During treatment with Klion-D 100, vaginal tablets, sexual intercourse should be avoided. In case of treatment failure, a replacement with another systemic trichomonadocidal and / or antifungal drug is recommended. In case of hypersensitivity, irritation of the mucous membranes, treatment with this drug should be discontinued. Interaction with other drugs Data on the interaction of metronidazole and miconazole nitrate with vaginal use are not known to date. When using Klion-D 100 vaginal tablets in combination with metronidazole tablets for oral administration, the following types of drug interactions may occur: Metronidazole can potentiate the anticoagulant effect of oral anticoagulants, which can lead to a prolongation of the prothrombin time, therefore, it is necessary to adjust their dose. Enzyme inducers (eg, phenytoin, phenobarbital) may increase the metabolism of metronidazole, which may result in decreased plasma levels and increased plasma clearance of phenytoin. Enzyme inhibitors (eg, cimetidine) may prolong the half-life and decrease the plasma clearance of metronidazole. The use of alcoholic beverages while taking metranidazole can cause disulfiram-like side effects (intestinal colic, nausea, vomiting, headaches and facial flushing). Metronidazole and disulfiram should not be taken together (there may be: an additive effect, psychotic reactions, confusion) Taking metronidazole may increase the content of lithium in the blood plasma, and therefore it is necessary to reduce the dose or discontinue the lithium preparation before starting metronidazole therapy. In cases of simultaneous use of cyclosporine and metronidazole, plasma concentrations of cyclosporine may increase. If combination therapy with these drugs is necessary, it is recommended to control plasma concentrations of cyclosporine. Metronidazole reduces the clearance of 5-fluorouracil and increases its toxicity. Metronidazole may affect the results of the assessment of certain biochemical parameters of the blood serum, such as aspartate aminotransferase (ACT), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), triglycerides and glucose hexokinase. ContraindicationsHypersensitivity to the active substances or any of the excipients of the drug. First trimester of pregnancy. Composition Each vaginal tablet contains: Active ingredients: metronidazole -100 mg, miconazole nitrate -100 mg , lactose monohydrate. Overdose The drug is intended for intravaginal use only. In case of inadvertent ingestion of a large amount of the drug, it is necessary to wash the stomach. If symptoms of intoxication appear in case of overdose (nausea, vomiting and ataxia), symptomatic therapy should be carried out, such as gastric lavage, activated charcoal, hemodialysis. There is no specific antidote. Metronidazole and its metabolites are well excreted by dialysis. Storage conditions Store at a temperature not exceeding 30 °C, in the original packaging to protect from light and moisture. Keep out of the reach of children. Buy Klion-D 100 vaginal tablets 100mg/100mg №10×1 Price for Klion-D 100 vaginal tablets 100mg/100mg №10×1
| INN | METRONIDAZOL+MICONAZOL |
|---|---|
| The code | 209 |
| Barcode | 5 997 001 383 384 |
| Dosage | 100mg/100mg |
| Active substance | metronidazole, miconazole |
| Manufacturer | Gedeon Richter Pls., Hungary |
| Importer | IOOO Interfarmaks 223028 Minsk region, Minsk district, Zhdanovichsky s / s, ag. Zhdanovichi, st. Star, 19a-5, room. 5-2 |
Related products
Women's health
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks