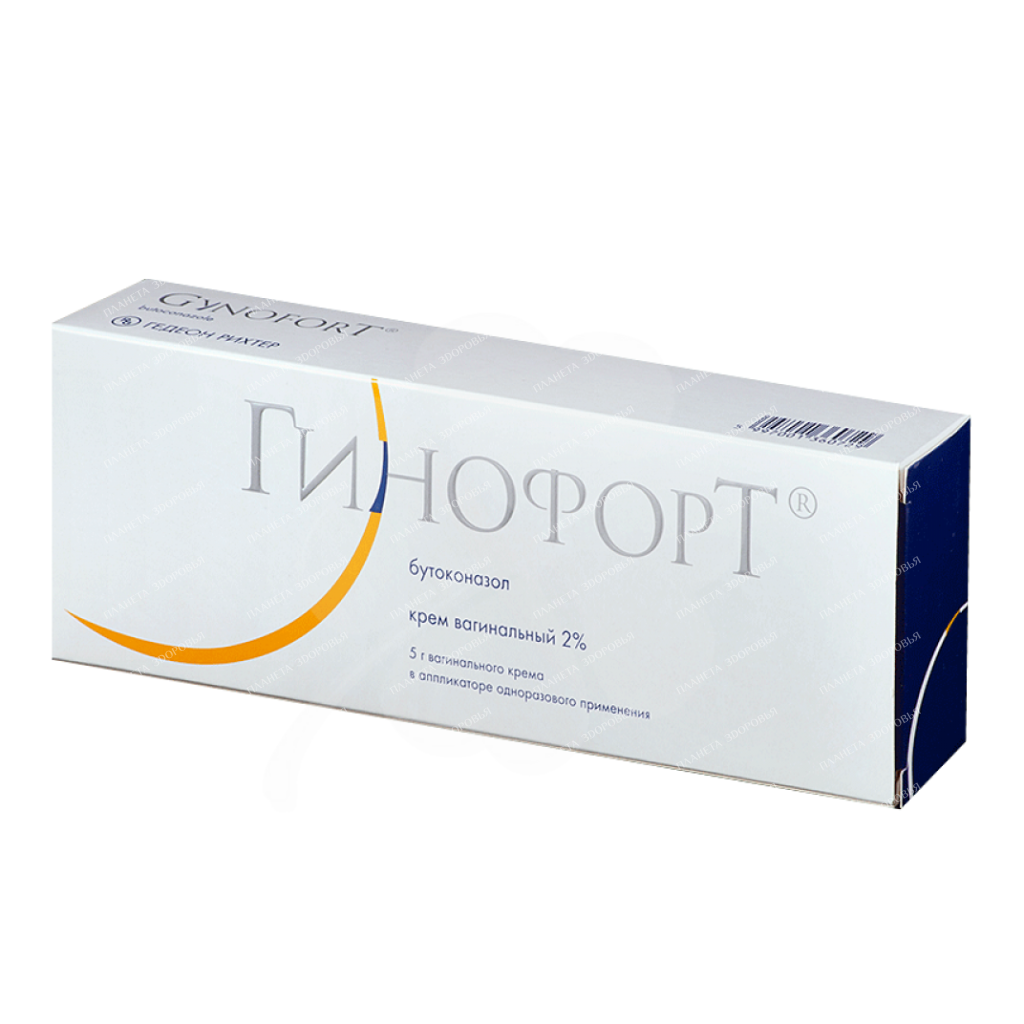
Gynofort vaginal cream 2% in applicator 5g №1
$39.00
Name:
Gynofort cream vag. 2% in app. 5g per pack No. 1
Description:
Soft homogeneous cream from white to almost white, free from foreign particles and without visible delamination Main active ingredient Butoconazole Product form Vaginal cream Dosage 5 g Indications for use Each pre-filled syringe applicator contains about 5 g of vaginal cream, which contains about 100 mg butoconazole nitrate, an active substance with antifungal activity. Gynofort® 2% Vaginal Cream is used for the topical treatment of vulvovaginal fungal infections caused by Candida albicans (a type of yeast) and other Candida species. Confirmation of the diagnosis by microbiological examination and / or bacteriological culture of a vaginal smear is recommended. Symptoms of a fungal infection include redness and swelling of the vulva, as well as itching, burning, and tenderness in the area. The infection may be accompanied by vaginal discharge, cheesy plaque, and pain when urinating or during intercourse. Dosage and administration Always strictly follow the doctor’s instructions for the use of Gynofort® 2% vaginal cream. If you are not sure about the correct use of the drug, consult your doctor. A single-dose, single-dose, cream-filled syringe applicator contains approximately 5 grams of vaginal cream. Dosing method: enter the entire contents of the syringe applicator into the vagina in one dose, preferably in the evening. Instructions for use of the disposable, pre-filled cream syringe applicator Step 1. Preparing the syringe applicator – Open the protective packaging and remove the syringe applicator filled with cream. – The syringe applicator is used when the tip is on. – Do not remove the tip. – Do not use the syringe applicator without a tip! – Do not heat the syringe applicator before use. While holding the syringe applicator firmly, pull on the ring to fully retract the plunger (see Figure 1). Step 2 Insert the syringe applicator into the vagina. – Gently insert the syringe applicator into the vagina as far as possible (see pictures 2 and 3). Step 3. Inserting the cream – While slowly depressing the plunger, squeeze the cream from the syringe into the vagina, then remove the empty syringe applicator from the vagina (see pictures 4, 5 and 6). The empty syringe must be disposed of. Gynofort® 2% vaginal cream is for intravaginal use only. If you accidentally swallow a vaginal cream, tell your doctor immediately. Use in children and adolescents The use of Gynofort® 2% vaginal cream is not recommended for children under 14 years of age due to the fact that the efficacy and safety of the drug has not been sufficiently studied in this age group. Prescribing the drug to adolescents at a sexually active age (14-18 years) requires an assessment of the risk-to-benefit ratio of the drug. If you missed the next application procedure Gynofort® Vaginal Cream 2% is a single-dose preparation. If the symptoms of the disease do not disappear, consult your doctor. If you stop using Gynofort® Vaginal Cream 2% is a single dose product. If the symptoms of the disease have not disappeared after a few days after using the drug, consult your doctor. If you have any further questions on the use of this drug, ask your doctor or pharmacist. Possible side effects Use during pregnancy and lactationConsult your doctor before starting the use of drugs. Pregnancy The use of Gynofort® 2% vaginal cream is not recommended for pregnant women during the first trimester of pregnancy, as well as for women of childbearing age who are sexually active, unless they use contraceptives. In the second and third trimesters of pregnancy, Gynofort® 2% vaginal cream can be used only on the recommendation of a doctor after a thorough analysis of the expected benefits compared with the possible risks of treatment. Breastfeeding It is currently unknown whether the active substance of the drug passes into breast milk. Therefore, you should consult your doctor before using a vaginal cream during breastfeeding. Precautions General Precautions If clinical symptoms persist despite treatment, laboratory tests should be repeated to confirm the original diagnosis and rule out conditions that may predispose to recurrence of vaginal yeast infection. Interactions with other drugs If you are currently taking or have taken any other drugs, including over-the-counter drugs, tell your doctor. At the same time, there is no information about the interaction of Gynofort® 2% vaginal cream with other drugs, with the exception of the mineral oil included in its composition, which can have a damaging effect on latex or rubber products. Composition- Active ingredient: 20 mg of butoconazole nitrate in 1 g of vaginal cream. – Excipients: sorbitol solution, liquid paraffin, glyceryl monoisostearate, polyglyceryl-3-oleate, microcrystalline wax, hydrophobic silicon dioxide, colloidal, disodium edetate, methyl parahydroxybenzoate, propyl parahydroxybenzoate, propylene glycol, purified water. Side effects Like all medicines, Gynofort® 2% vaginal cream may cause side effects in a limited number of patients. Based on the results of clinical studies and post-marketing reports, the following complaints are most often reported (? 5%): burning sensation in the vagina, itching, inflammation and swelling, pain in the pelvis or lower abdomen, cramps in the lower abdomen, or a combination of two or more of these symptoms . Potential local side effects that occur with the use of Gynofort® 2% vaginal cream are very similar to the symptoms of a vulvovaginal fungal infection. If symptoms do not disappear during treatment, contact your doctor as soon as possible. Allergic reactions (hypersensitivity reactions) can occur with any medication. Therefore, if you experience symptoms of an allergic reaction such as redness or peeling of the skin, skin or mucous membrane lesions, or an allergic rash (urticaria), contact your healthcare provider as soon as possible. If the severity of side effects increases, or you notice additional side effects not listed in this leaflet, contact your doctor. Storage conditionsStore at a temperature not exceeding 25 °C. Avoid storage above 25°C. Keep the drug out of the reach of children. Shelf life – 3 years. Do not use after the expiry date stated on the packaging. The expiration date includes the last day of the specified month. Do not dispose of the drug in the sewer or with household waste. Ask your pharmacist how to dispose of unwanted medication. Such measures will help in the fight for the preservation of the environment. Buy Gynofort vaginal cream 2% in applicator 5g №1 Price for Gynofort vaginal cream 2% in applicator 5g №1
| INN | BUTOKONAZOL |
|---|---|
| The code | 48 594 |
| Barcode | 5 997 001 360 729 |
| Dosage | 2% 5g |
| Active substance | Butoconazole |
| Manufacturer | Gedeon Richter Pls., Hungary |
| Importer | IOOO Interfarmaks 223028 Minsk region, Minsk district, Zhdanovichsky s / s, ag. Zhdanovichi, st. Star, 19a-5, room. 5-2 |
Related products
Antifungals & Antiparasitics
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks