Name:
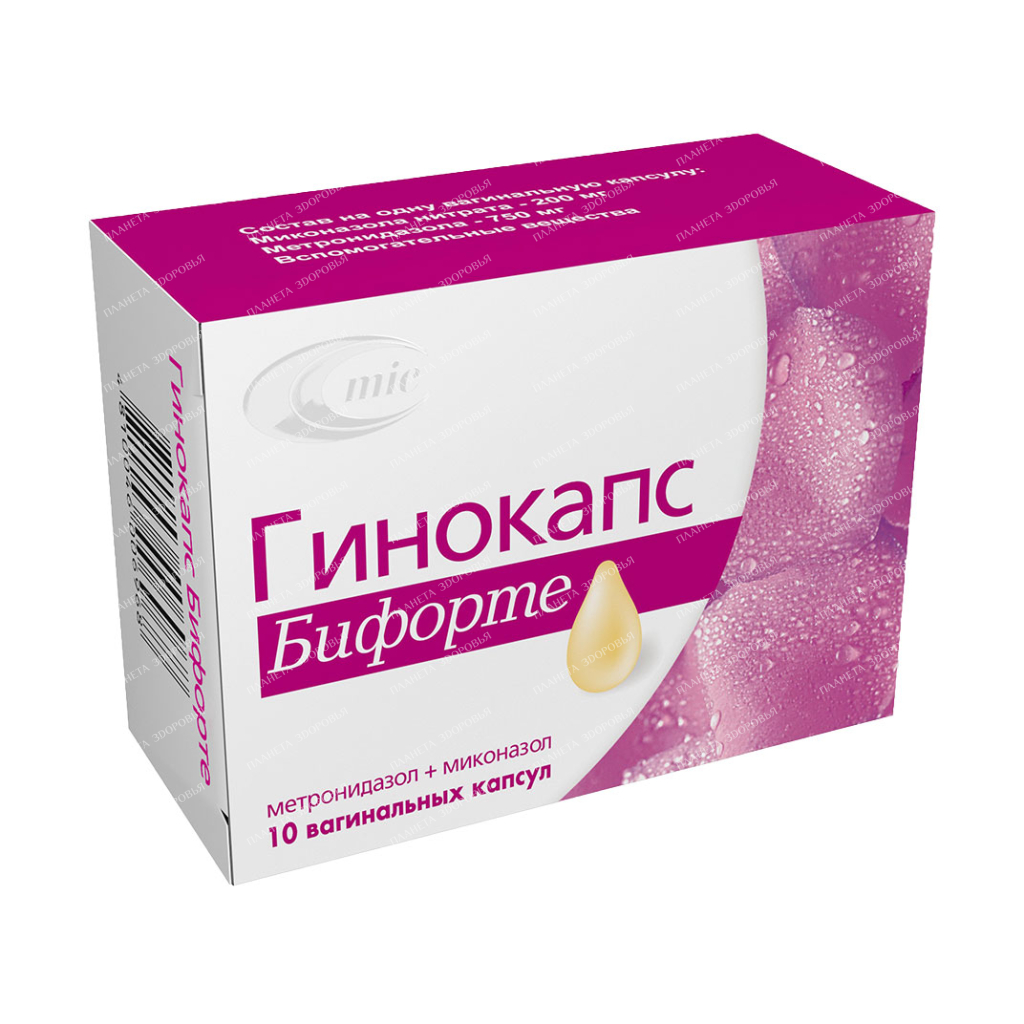
Gynocaps biforte
Description:
Capsules are soft gelatinous ovoid-shaped with a pointed end, with a seam, elastic, opaque, almost white or light beige. The main active substance Metronidazole + miconazole Release form Vaginal capsules. 5 capsules in a blister pack, 2 or 3 blister packs together with a leaflet in a pack. Dosage 200 mg + 750 mg Special instructions During treatment with the drug and for at least three days after treatment, the use of alcohol is prohibited (disulfiram-like reactions may develop: spastic abdominal pain, nausea, vomiting, headache, sudden flushing of the face) and abstinence is recommended from sexual intercourse. In the treatment of trichomoniasis, it is advisable to simultaneously treat the sexual partner with metronidazole tablets for oral administration. It is advisable to prescribe vaginal capsules in combination with oral forms of metronidazole. In the case of using the drug in conjunction with metronidazole for oral administration, especially with a second course, it is necessary to control the picture of peripheral blood (danger of leukopenia). With caution: pregnancy (Il-Ill trimesters), history of leukopenia. During therapy, other vaginal agents (tampons, syringe, spermicidal agents) should not be used. It is possible to change the results when determining the level of liver enzymes, glucose (hexokinase method), theophylline and procainamide in the blood. High doses and long-term systemic use of metronidazole can cause peripheral neuropathy and epilepsy. Do not swallow or otherwise apply! Indications for use Ginocaps Biforte is used for local treatment of infectious diseases of the vagina: bacterial vaginosis; trichomoniasis; candidal vaginitis; mixed vaginal infection. Method of application and doses Ginocaps Biforte is used intravaginally, 1 vaginal capsule is injected deep into the vagina for 7-10 days at night. For recurrent vaginitis or vaginitis resistant to other treatment: 1 vaginal capsule for 14-15 days. For the treatment of trichomonas vaginitis, it is advisable to combine vaginal Ginocaps Biforte capsules with oral forms of metronidazole or other systemic trichomonacid preparations. At the same time, it is necessary to treat the sexual partner with metronidazole tablets or other systemic trichomonacid drugs. Children: not recommended for children. Patients with renal / hepatic insufficiency: Renal insufficiency: the half-life of metronidazole does not change. Dose reduction is not required. However, in severe cases requiring hemodialysis, dose adjustment is necessary. In cases of severe hepatic insufficiency, the clearance of metronidazole may be impaired. At high levels of metronidazole in blood plasma, an increase in the symptoms of encephalopathy can be observed, so metronidazole should be used with caution in patients with hepatic encephalopathy. The daily dose in patients with hepatic encephalopathy should be reduced to 1/3. Application during pregnancy and lactationGinocaps Biforte capsules can be used after the first trimester of pregnancy under medical supervision, provided that the expected benefit to the mother outweighs the potential risk to the fetus. At the time of treatment, breastfeeding should be stopped, since metronidazole passes into breast milk. Breastfeeding can be resumed 24-48 hours after the end of treatment. Precautions Use of the drug in elderly patients Use in elderly patients requires caution due to age-related changes in the pharmacokinetics of metronidazole. Influence on the ability to drive a car, work with machinery Does not affect. Interaction with other drugs The drug may enhance the effect of oral anticoagulant drugs. Prothrombin time may increase, so dose adjustment of oral anticoagulants is necessary. Enzyme inducers (eg: phenytoin, phenobarbital) can accelerate the elimination of metronidazole, which will lead to a decrease in its plasma level with a simultaneous increase in the clearance of phenytoin. Enzyme inhibitors (eg: cimetidine) may increase the half-life, reduce the clearance of metronidazole. The simultaneous use of alcohol causes reactions similar to disulfiram (cramping abdominal pain, nausea, vomiting, headache, redness of the skin). Combined use with disulfiram is unacceptable (additive action, can cause a psychotic state, confusion). The level of lithium in the blood may increase during the course of treatment with metronidazole, therefore, before starting the use of Ginocaps Biforte, it is necessary to reduce the dose of lithium or stop taking it for the duration of treatment. Simultaneous administration of cyclosporine with metronidazole can lead to an increase in the level of cyclosporine in plasma, which requires laboratory monitoring (determination of the level of cyclosporine in blood plasma). Metronidazole reduces the clearance of 5-fluorouracil, and therefore increases its toxicity. Metronidazole and miconazole inhibit the metabolism of astemizole and terfenadine and increase their plasma concentration. The drug may interfere with the determination of the activity of ALT, ACT, lactate dehydrogenase, glucose-hexokinase and triglyceride levels in laboratory studies. Contraindications severe liver dysfunction; epilepsy; I trimester of pregnancy, lactation; hypersensitivity to the components of the drug; porphyria; in patients who drink alcohol during treatment or within 3 days after the end of treatment; in patients taking disulfiram during treatment or planning to use it within 2 weeks after the end of treatment. Composition One capsule contains: metronidazole – 750 mg, miconazole nitrate – 200 mg. Excipients – beeswax, miglyol 812 N. The composition of the gelatin capsule shell: gelatin, glycerin, purified water, methyl parahydroxybenzoate E-218, propyl parahydroxybenzoate E-216, titanium dioxide E-171. Overdose There are no data on overdose with intravaginal use of metronidazole. After intravaginal administration, metronidazole may be absorbed in sufficient amounts to cause systemic effects. Symptoms: nausea, vomiting, abdominal pain, diarrhea, generalized itching, metallic taste in the mouth, movement disorders (ataxia), dizziness, parestension, convulsions, peripheral neuropathy (including after prolonged use in high doses), leukopenia, dark urine. Treatment: in case of accidental ingestion, if necessary, gastric lavage can be performed. There is no specific antidote. Symptomatic and supportive therapy is recommended. Side effects Local: rarely – irritation of the vaginal mucosa (burning, itching). If irritation is severe, treatment should be discontinued. It is possible to develop systemic effects: From the gastrointestinal tract: nausea, vomiting, loss of appetite, cramping pain in the lower abdomen, diarrhea, furred tongue, bitter, metallic taste in the mouth. From the hematopoietic system: reversible neutropenia (leukopenia). From the side of the central nervous system: peripheral neuropathy (feeling of numbness of the extremities), rarely and only with prolonged use, headaches, convulsions, drowsiness, dizziness, impaired coordination, ataxia, confusion occur. Allergic reactions: skin rash, urticaria, skin itching, erythema multiforme exudative, angioedema and anaphylactic reaction were very rare. The effect of the drug on the liver: rarely reported increased activity of liver enzymes, cholestasis, jaundice. Others: fever, dark urine (causes a metabolite of metronidazole, has no clinical significance). These side effects are very rare due to the low concentration of metronidazole in the blood during intravaginal use of Ginocaps Biforte vaginal capsules. Miconazole nitrate is not absorbed after the introduction of Ginocaps Biforte vaginal capsules into the vagina. If side effects occur, treatment with Ginocaps Biforte should be discontinued. Storage conditions Store in a place protected from moisture and light at a temperature of 15 ° C to 25 ° C. Store in a place inaccessible to children. Shelf life 18 months. Do not use after the expiry date stated on the packaging. Buy Ginocaps BIFORTE vaginal capsules 200mg/750mg №5×2
| INN | METRONIDAZOL+MICONAZOL |
|---|---|
| The code | 106 915 |
| Barcode | 4 810 046 006 968 |
| Dosage | 200mg/750mg |
| Active substance | metronidazole, miconazole |
| Manufacturer | Minskintercaps UP, Belarus |
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks