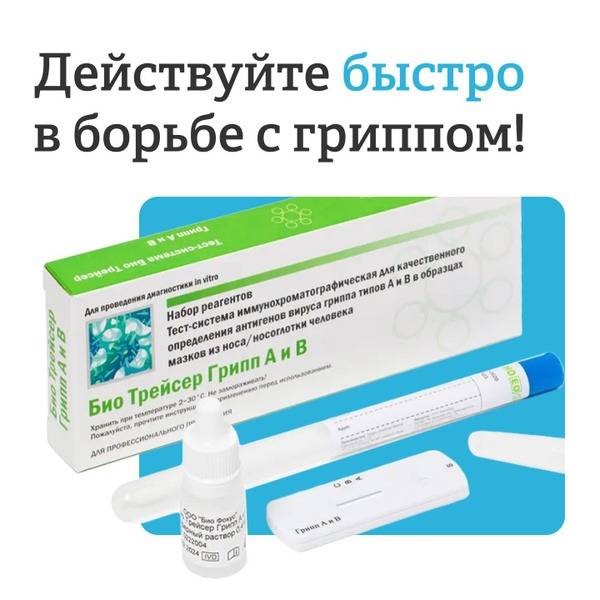
The BioTracer Influenza A & B Antigen Rapid Test is a single-use, qualitative immunochromatographic assay for the rapid detection of influenza A (including subtypes H1N1 and H3N2) and/or B virus antigens directly from human nasal/nasopharyngeal swab specimens. This test provides results in just 10-15 minutes, facilitating quick diagnosis and differentiation of influenza types.
The BioTracer test utilizes a qualitative immunochromatographic assay. If influenza A and/or B virus antigens are present in the sample, they bind to a specific conjugate (monoclonal antibodies to influenza A and B antigens labeled with colloidal gold) in the sample application area. This "antigen-antibody conjugate" complex migrates along the membrane.
In the test zone, the complex interacts with immobilized monoclonal antibodies specific to influenza A antigen (test zone A) and/or influenza B antigen (test zone B), forming a colored "substrate antibody/antigen/antibody conjugate" complex. A colored line indicates a positive result; the absence of a line indicates a negative result.
Unreacted conjugate interacts with polyclonal anti-IgG antibodies in the control zone (zone C), forming a colored immune complex. The appearance of a red or purple control line (C) confirms proper test procedure and result validity. This line always appears regardless of the presence of influenza antigens.
Influenza A, Influenza B, rapid test, antigen test, flu test, immunochromatographic assay, nasal swab, nasopharyngeal swab, rapid influenza diagnostic test, point-of-care testing, POC testing, BioTracer, Bio Focus LLC, H1N1, H3N2, flu diagnosis, influenza detection, single-use test, quick flu test, home flu test.