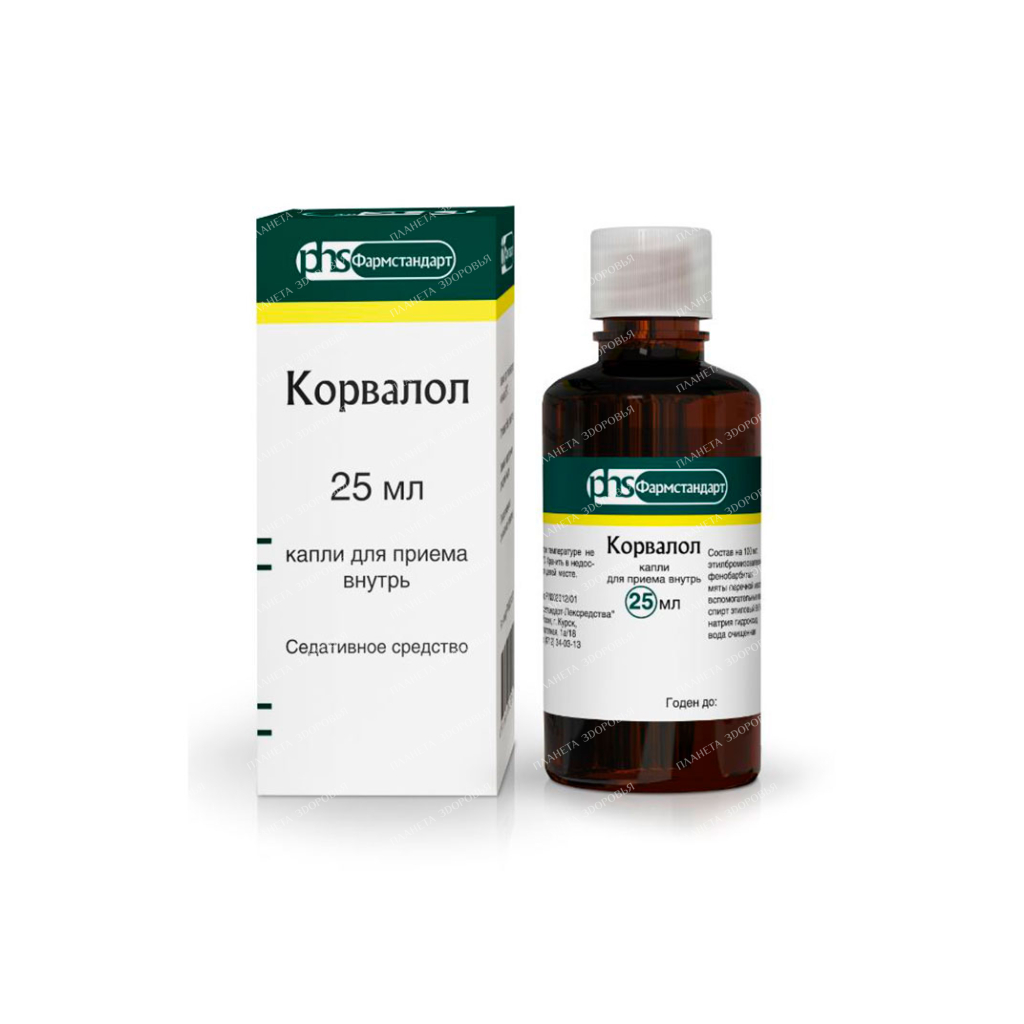
Corvalol-Pharmstandard drops for oral administration in 25ml №1
$9.00
Corvalol-Pharmstandard Drops: A Comprehensive Guide
Corvalol-Pharmstandard drops are a widely-used sedative medication containing a blend of peppermint oil, phenobarbital, and ethyl bromoisovalerianate. This potent combination provides relief from functional disorders of the nervous system, including neurasthenia and sleep disturbances. This comprehensive guide delves into the uses, benefits, risks, and considerations associated with Corvalol-Pharmstandard drops.
What are Corvalol-Pharmstandard Drops?
Corvalol-Pharmstandard drops are a transparent, colorless liquid with a distinctive odor. Each milliliter (26 drops) contains:
- Ethyl ester of α-bromoisovaleric acid: 20 mg
- Phenobarbital: 18.26 mg
- Mint oil: 1.42 mg
The drops also contain ethyl alcohol (ethanol 96%), sodium isovalerianate as a stabilizer, and purified water.
Why are Corvalol-Pharmstandard Drops Used?
Corvalol-Pharmstandard drops are prescribed as a symptomatic remedy for:
- Functional disorders of the nervous system: Neurasthenia (a condition characterized by fatigue, irritability, and sleep problems)
- Sleep disturbances: Insomnia and difficulty falling asleep
Important Considerations:
- Short-term use: Due to the risk of addiction and dependence, Corvalol-Pharmstandard drops are intended for short-term use only.
- Alcohol content: The drops contain a significant amount of ethyl alcohol (at least 56% v/v), which should be considered by individuals with alcohol dependence, pregnant women, breastfeeding mothers, and children under 18.
- Potential side effects: Corvalol-Pharmstandard drops can cause a range of side effects, including drowsiness, dizziness, headache, and confusion. It’s crucial to be aware of these risks and consult a healthcare professional immediately if any adverse effects arise.
How to Use Corvalol-Pharmstandard Drops:
Dosage and duration of administration are determined by a doctor on an individual basis. Adults typically take 15-30 drops (dissolved in water) before meals, 2-3 times daily.
Who Should Avoid Corvalol-Pharmstandard Drops?
Corvalol-Pharmstandard drops are contraindicated in the following cases:
- Hypersensitivity: Allergy to bromine or any component of the drug.
- Renal or Liver Failure: Pre-existing kidney or liver dysfunction.
- Porphyria: A rare genetic disorder affecting heme production.
- Alcoholism: Individuals with alcohol dependence.
- Epilepsy: Patients with epilepsy.
- Traumatic brain injury: Individuals with traumatic brain injury or other brain diseases with a decreased seizure threshold.
- Pregnancy and Lactation: Pregnant or breastfeeding women.
- Children under 18: Not recommended for children and adolescents.
Potential Drug Interactions:
Corvalol-Pharmstandard drops can interact with various medications, including:
- Central nervous system depressants: Alcohol, sedatives, and other drugs that depress the central nervous system should be avoided.
- Coumarin derivatives: Increased bleeding risk may occur.
- Lamotrigine: May reduce the effectiveness of lamotrigine (anticonvulsant medication).
- Thyroid hormones: May reduce the effectiveness of thyroid hormones.
- Doxycycline: May interfere with the absorption of doxycycline (antibiotic).
- Chloramphenicol: May increase the risk of side effects associated with chloramphenicol (antibiotic).
- Antifungal drugs (azole type): May increase the risk of side effects associated with azole antifungal drugs.
- Griseofulvin: May increase the risk of side effects associated with griseofulvin (antifungal medication).
- Glucocorticoids: May increase the risk of side effects associated with glucocorticoids.
- Oral contraceptives: May reduce the effectiveness of oral contraceptives.
- Methotrexate: May increase the toxicity of methotrexate (chemotherapy drug).
- Phenytoin: May interfere with the metabolism of phenytoin (anticonvulsant medication).
- Sodium valproate and valproic acid: May inhibit the metabolism of phenobarbital.
- Drugs that cause a disulfiram-like reaction: Alcohol should be avoided due to the potential for adverse reactions.
- Other medications containing ethyl alcohol: Avoid simultaneous use.
Always consult your doctor before taking Corvalol-Pharmstandard drops, especially if you are taking other medications.
Storage and Shelf Life:
Store Corvalol-Pharmstandard drops in a cool, dark place, out of reach of children. The shelf life is 2 years and 6 months from the date of manufacture.
Conclusion:
Corvalol-Pharmstandard drops offer potential relief for functional disorders of the nervous system and sleep disturbances. However, it’s essential to use this medication cautiously and under the guidance of a healthcare professional due to its potential side effects, interactions, and risks associated with addiction and dependence. This comprehensive guide provides vital information for informed decision-making regarding Corvalol-Pharmstandard drops. Remember, always prioritize your health and safety and consult your doctor before making any decisions about your medication.
| INN | PEPPERMINT OIL + PHENOBARBITAL + ETHYL BROMISOVALERIANATE |
|---|---|
| The code | 1 899 |
| Barcode | 4 601 669 002 051 |
| Dosage | 25ml |
| Active substance | Ethyl ester alpha-bromizovaler. to-you, phenobarbital, sodium hydroxide, peppermint oil, ethyl. alcohol |
| Vacation rate | 1 |
| Manufacturer | Pharmstandard-Leksredstva OJSC, Russia |
| Importer | IOOO Interfarmaks 223028 Minsk region, Minsk district, Zhdanovichsky s / s, ag. Zhdanovichi, st. Star, 19a-5, room. 5-2 |
Related products
Neurological Health
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks