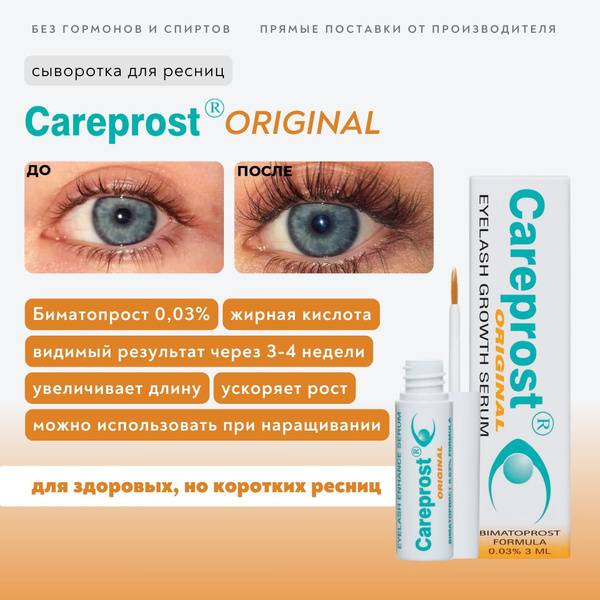
Experience dramatic eyelash enhancement with Careprost Original Eyelash Growth Serum, featuring the clinically proven active ingredient Bimatoprost 0.03%. Designed for those seeking noticeably longer and fuller lashes, Careprost Original stimulates hair follicles, resulting in up to a 200% increase in lash length and volume. See visible improvements within 3-4 weeks of daily application. A full treatment course is typically 3 months.
Careprost Original's key ingredient, Bimatoprost 0.03%, is a fatty acid that promotes healthy circulation, accelerating hair follicle function and focusing on the anagen (growth) phase for maximized length. While mild irritation may occur initially, this is a normal reaction to the active ingredients. Bimatoprost is a physiologically active fatty acid previously used in popular Indian eyelash growth serums.
Careprost Original prioritizes both growth and lash health. Our unique formula incorporates the BeauPlex® VH vitamin complex (Vitamins E, B3, B5, B6, and stabilized Vitamin C), providing powerful antioxidant protection and hydration to prevent premature aging and enhance pigment. We've also included Sensicare C 2050, a next-generation natural preservative compliant with ISO 16128 standards, to gently hydrate and soothe delicate eyelid skin.
Careprost Original is compatible with various beauty treatments, including eyelash extensions and lamination. We prioritize transparency and user trust; therefore, our product documentation, including the Declaration of Conformity and quality passport, is readily available. Careprost Original is not a medical product and is free from harsh ingredients such as alcohol, hormones, and prostaglandins.
Apply Careprost Original along the upper lash line as close to the roots as possible, focusing on the follicle area. It can be used before makeup and is compatible with lash extensions, lamination, and Botox treatments. It's also suitable for eyebrow growth and can be used after eyebrow tattooing, microblading, tinting, and lamination. Mild irritation may occur at the application site but typically subsides upon discontinuation.





