Sterile Beige Latex Surgical Gloves – ProFeel DHD Platinum PF Size 7.5 (5 Pairs)
$29.00
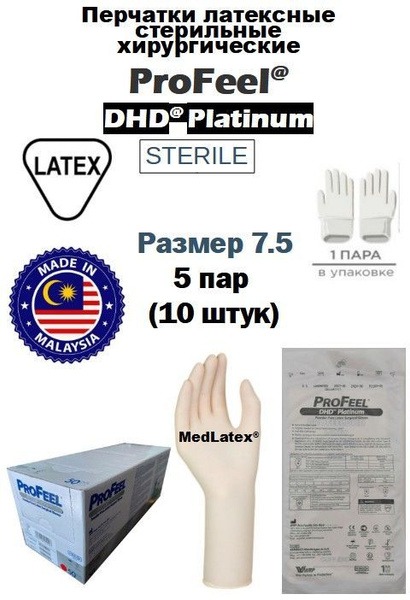
ProFeel DHD Platinum Latex PF Sterile Surgical Gloves – Beige, Size 7.5 (10 Count)
Product Description
ProFeel DHD Platinum Latex PF sterile surgical gloves are designed to meet the rigorous demands of modern healthcare professionals. These powder-free gloves eliminate the risk of allergic reactions, powder-related adhesions, and the need for pre-procedure dusting. Experience unparalleled comfort and dexterity with their exceptional tactile sensitivity, minimizing hand fatigue during extended procedures.
The innovative Damp-hand-Donnable (DHD) design ensures a secure and easy fit, even with wet hands, reducing the risk of tearing and slippage. Their consistent physical properties, maintained even after prolonged storage, guarantee reliable performance throughout their shelf life. The micro-textured surface provides superior grip in both wet and dry conditions.
Key Features & Benefits
- Natural Latex: Made from high-quality natural latex for optimal comfort and performance.
- Powder-Free: Eliminates the risk of latex allergies and powder-related complications.
- Damp-hand-Donnable (DHD): Easy to don even with wet hands.
- Micro-Textured Surface: Provides excellent grip in wet and dry conditions.
- Beaded Cuff: Ensures a secure fit and prevents slippage.
- Polymer Coating: Enhances comfort and reduces friction.
- Superior Tactile Sensitivity: Allows for precise control and dexterity.
- Gamma Sterilized: Ensures sterility and safety.
- Exceptional Comfort and Fit: Feels like a “second skin.”
- Durable and Reliable: Maintains its integrity and performance.
Specifications
- Size: 7.5
- Quantity: 10 gloves (5 pairs)
- Color: Beige
- Thickness: Finger: 0.21mm, Palm: 0.19mm, Cuff: 0.15mm
- Surface: Smooth exterior with micro-texturing; Polymer-coated interior.
- Grip Level: Medium
- Certifications: CE MH Class IIa & PPE Category III, AS/NZS 4179, EN 16523-1, EN 374 (Microorganisms), EN 374 (Low Chemical Resistance), EN 420, EN 420:2003 + A1:2009, EN 455-1, EN 455-2, EN 455-3, EN 455-4, EN ISO 374-1:2016 type B KPT, EN ISO 374-5:2016 VIRUS, ISO 10282, JIS T9107, EN 556, ISO 11137-1, ISO 13485, ISO 14001, ISO 9001, Korean GMP, AQL 0.65
 Free worldwide shipping on orders $99+
Free worldwide shipping on orders $99+  US: temporary delays — postal services aligning new import rules,
US: temporary delays — postal services aligning new import rules,  EU: 1–2 weeks,
EU: 1–2 weeks,  Worldwide: 1–4 weeks
Worldwide: 1–4 weeks